
Indicates that an atom has a central solid core called as nucleus surrounded by the electronsĭoes not give any idea about constituents of nucleus Indicates that atom is spherical in shape States that electrons are located around a central solid material States that electrons are uniformally distributed in an atom States that an atom is composed of an atomic nucleus around which electrons are revolving in an orbitĭoes not give any detail about the atomic nucleus Most of atom's mass is in the nucleus.States that electron are embedded in a positively charged solid material which is spherical in shape.Only on the number of protons in the nucleus. The important point here is that chemical and physical properties of matterĭepend only on the electron cloud surrounding the atoms. Physical properties of matter include hardness, malleability, A typical number of atoms in a piece of matter on a human scale In molecules form the domain of chemistry.Ī solid consists of a regular array or lattice containing a very large number The arrangements and rearrangements of atoms Many cases approximately equal to the number of protons.Ī small number of atoms may combine to form a molecule such as water (H 2O) The number of neutrons in the nucleus is in It is probable that most of the matter in the However, in the sun and the stars most of the On earth most of the matter we come inĬontact with is made of atoms. It is possible to have a system with N e not equal to N p,Ĭalled an atom it is called an ion. In an atom the number of electrons equals the number of protons, N e Than the repulsive electric force and so nuclei are held together. Neutrons, and between a neutron and proton. Nuclear force, that is mostly attractive and acts between two protons, between two Is the nucleus held together? There is another force, called the Repel each other, and the neutrons don't feel any electric force. Inside the nucleus all the electric forces are repulsive because the protons The electron jumps from one state to another when it receives or emits a quantum of energy in the form of light (or other form of electromagnetic radiation). The electron can exist only in one of a discrete set of "energy states", and the lowest energy state is stable. But the reason for the atom's stability - the fact that the electron's orbit does not collapse - lies in the fundamental nature of quantum mechanics, the science that supersedes Newton's mechanics in the world of the atom. Why don't the electrons fall into the nucleus under the influence of thisįorce? The atom looks superficially like the solar system, with its planets in orbit around the central sun.

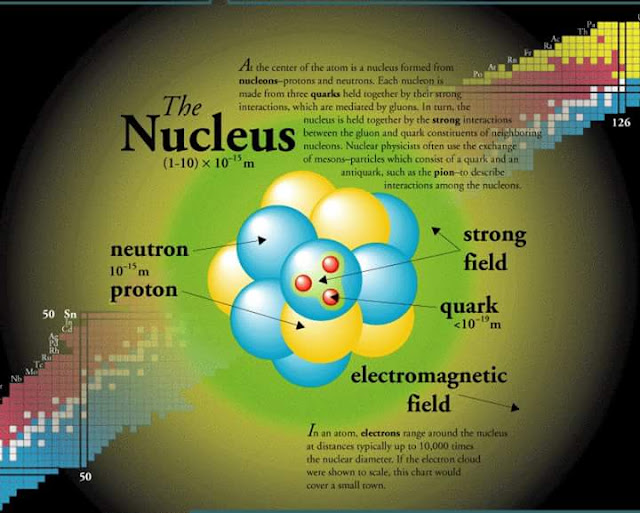
In other words, atoms would not exist if it were not for the electric force. The nucleus is aĭense ball of positive charge in the center of the atom and it exerts anĪttractive force on the electrons, thereby holding them as part of the atom. Like charges repel and unlike charges attract. The electron has a negative electric charge Įlectric charge of exactly the same strength the neutron has no electricĬharge. That is why the nucleus has most the atom's mass. These masses are more than 2000 times the mass of the electron.

Proton and neutron have almost the same mass - the neutron's is slightly The proton and neutron are spherical, about Size is not known, but we do know that it is smaller than a nucleus),īut it occupies the space of the atom by constantly whirling around in The outer volume of the atom (which means most of The nucleus is made up of protons and neutrons bound together byĪttractive forces. In shape, although some are spheroidal (egg-shaped). (Hence the numbers given above for masses ofĪtoms also apply approximately to the nucleus alone.) Nuclei are usually spherical Typically the nucleus contains more than 99.9% Typical for the smaller nuclei larger ones go up to about 10 timesĪlthough it is very small, the nucleus is massive compared to A good comparison of the nucleus to the atom is like a pea in the middle of a racetrack. The nucleus of an atom is about 10 -15 m in size this means it is aboutġ0 -5 (or 1/100,000) of the size of the whole atom. The masses of other atoms go up to about 200 times this. The smallest mass is the hydrogen atom its mass is about 10 -27 kg. Spherical in shape, although they are not always so. It is also a good approximation to think of atoms as Atoms of different elements areĭifferent sizes, but 10 -10 m can be thought of as a rough value forĪny atom. Means a row of 10 8 (or 100,000,000) atoms would stretchĪ centimeter, about the size of your fingernail. The atom is about 10 -10 meters (or 10 -8 centimeters) in size.


 0 kommentar(er)
0 kommentar(er)
